This article is part of the Heat Transfer in Process Plants
series, which builds the core physical understanding engineers rely on
before moving into design tools.
It follows the earlier discussion on the fundamental reason heat transfer
occurs in:
Why Heat Transfer Always Occurs
.
This article clarifies the difference between heat and temperature in plain,
practical terms — without unnecessary textbook jargon.
A Difference That Quietly Controls Plant Performance
Heat and temperature are often used interchangeably in everyday language.
In process plants, this confusion is not harmless.
Many operating issues, design misunderstandings, and troubleshooting failures can be traced back to one simple problem: treating heat and temperature as the same thing.
They are related, but they are not identical.
Understanding the difference is essential for anyone trying to understand how a plant actually behaves.
This article explains that difference clearly, without formulas, without academic language, and without unnecessary theory — focusing only on how plants respond in the real world.
Table of Contents
Why This Difference Matters More Than It Appears
Plants rarely fail because someone did not know an equation.
They fail because assumptions were wrong.
One of the most common wrong assumptions is:
- “If the temperature is correct, heat transfer must be fine.”
In reality:
- temperature can look correct while heat transfer is insufficient,
- temperature can look alarming while actual heat content is low,
- stable temperature does not always mean stable operation.
This is why understanding heat versus temperature is not optional knowledge.
It is foundational.
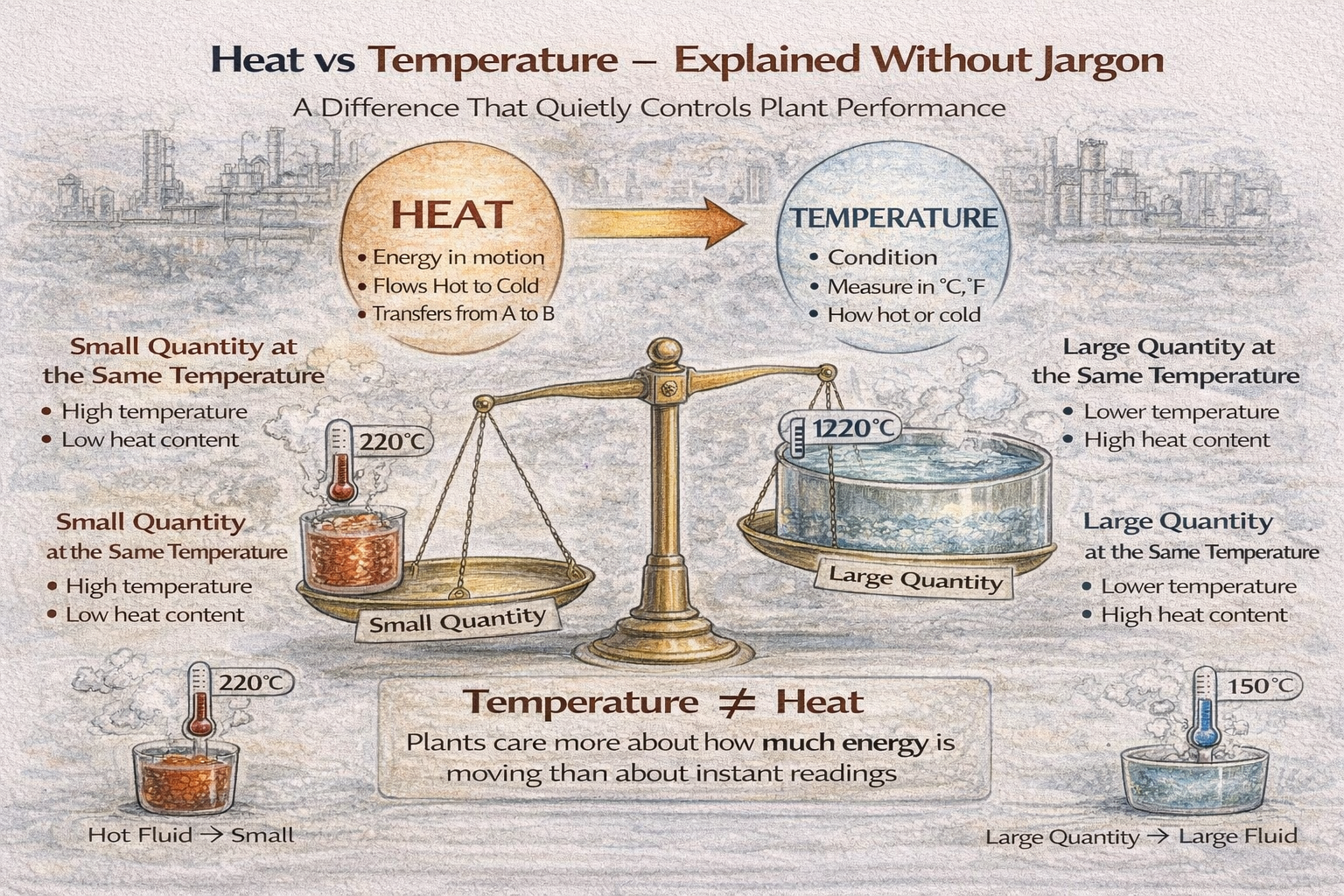
Temperature Is a Condition, Not an Action
Temperature tells us how hot or cold something is.
That is all it does.
It does not tell us:
- how much energy is present,
- how fast energy is moving,
- how much energy can be transferred.
Two objects can have the same temperature and behave very differently in a plant.
For example:
- a thin metal pipe at 200°C,
- a large vessel containing several tons of liquid at 200°C.
The temperature number is the same.
The thermal reality is completely different.
Heat Is Energy in Motion
Heat is energy moving because of temperature difference.
Heat is not something an object “has”.
It is something that flows.
Whenever energy moves from a hotter region to a colder region, heat transfer is taking place.
This movement:
- has direction,
- has rate,
- has consequences.
In plants, it is the rate of heat movement, not the temperature itself, that determines:
- whether a reactor stays under control,
- whether a product reaches target conditions,
- whether equipment performs as expected.
Same Temperature, Very Different Heat Content
This is where confusion causes real problems.
Consider these situations:
- A small quantity of hot fluid
- A large quantity of warm fluid
The hot fluid may show a higher temperature.
The warm fluid may contain far more total energy.
Operators often react to temperature readings because they are visible and immediate.
But equipment responds to energy balance, not just temperature.
This is why:
- large systems respond slowly to heating or cooling,
- small systems change temperature rapidly,
- temperature spikes do not always indicate dangerous energy levels,
- steady temperature does not guarantee safe heat removal.
Why Plants Care More About Heat Than Temperature
Temperature is easy to measure.
Heat is harder to visualize.
But plants behave according to heat flow.
For example:
- a reactor may show acceptable temperature,
- yet insufficient heat removal slowly accumulates energy,
- leading to delayed instability.
Or:
- a heat exchanger may deliver correct outlet temperature,
- but requires excessive utility flow,
- increasing operating cost quietly.
In both cases, temperature looks acceptable.
Heat transfer efficiency does not.
Temperature Can Be Misleading
In many plant situations, temperature readings provide only part of the picture.
Examples:
- wall temperature differs from bulk fluid temperature,
- surface temperature hides internal gradients,
- sensor location affects perceived stability,
- averaging masks local hot spots.
Plants that rely only on temperature readings often:
- react late,
- misdiagnose issues,
- apply short-term fixes.
Understanding heat transfer allows engineers to interpret temperature correctly rather than blindly trusting numbers.
Heat Transfer Explains Why Bigger Systems Behave Differently
One of the first surprises for new engineers is this:
Large equipment does not behave like small equipment.
Heating or cooling a laboratory vessel is quick.
Heating or cooling a large plant vessel is slow.
The reason is not temperature.
It is heat content and heat transfer rate.
Large systems:
- contain more energy,
- require more heat transfer surface,
- respond more slowly,
- retain heat longer.
This is why:
- startups take time,
- shutdowns require caution,
- transient conditions create stress.
Temperature alone does not explain this behavior.
Heat balance does.
Heat Explains Why Fouling Hurts Performance
Fouling does not change temperature setpoints.
It changes heat transfer resistance.
As fouling builds:
- the same temperature difference transfers less heat,
- more utility is required,
- approach temperatures increase,
- margins shrink.
Plants often respond by:
- increasing steam,
- increasing cooling water,
- pushing equipment harder.
Temperature may still meet targets.
Efficiency quietly degrades.
This slow erosion is invisible without understanding heat transfer.
Why Seasonal Changes Affect Plants
Many plants behave differently in summer and winter.
This is not because chemistry changes.
It is because heat transfer conditions change.
Ambient temperature affects:
- heat losses,
- cooling efficiency,
- utility return temperatures,
- condensation behavior.
Temperature readings shift slightly.
Heat balance shifts significantly.
Plants designed with narrow thermal margins often struggle seasonally — not because of control failure, but because heat transfer assumptions were optimistic.
Heat Transfer Explains Thermal Inertia
Thermal inertia is the resistance of a system to temperature change.
It depends on:
- mass,
- specific heat,
- heat transfer rate.
High thermal inertia systems:
- resist quick temperature changes,
- respond slowly to control actions,
- store large amounts of energy.
Understanding this helps explain:
- delayed response after valve changes,
- overshoot during startups,
- long stabilization times.
Temperature control without heat understanding often leads to aggressive tuning and instability.
Why Control Systems Depend on Heat, Not Just Temperature
Control loops act on temperature signals.
But the process responds to heat transfer.
If heat transfer is slow:
- control actions appear ineffective,
- operators overcorrect,
- oscillations develop.
If heat transfer suddenly improves:
- temperature responds rapidly,
- overshoot occurs,
- safety margins reduce.
Effective control requires understanding how fast heat can actually move through the system.
Owners Often See the Cost Impact First
From an ownership perspective, confusion between heat and temperature leads to:
- higher energy bills,
- oversized utilities,
- frequent operational intervention,
- unexplained efficiency losses.
Plants that focus only on temperature compliance often miss:
- wasted energy,
- declining exchanger effectiveness,
- increasing operating cost.
Understanding heat allows owners to ask better questions and recognize early signs of inefficiency.
Why This Concept Comes Before All Calculations
Before discussing:
- heat exchanger sizing,
- coefficients,
- LMTD,
- simulation outputs,
one must clearly understand:
- temperature is an indicator,
- heat is the driver.
Without this clarity:
- equations feel disconnected,
- software results mislead,
- troubleshooting becomes reactive.
With this clarity:
- numbers make sense,
- trends become meaningful,
- decisions improve.
Final Perspective
Temperature is what plants show you.
Heat is what plants respond to.
Confusing the two leads to:
- wrong conclusions,
- inefficient operation,
- repeated problems.
Understanding the difference does not require advanced mathematics.
It requires careful thinking about how energy actually moves.
Once this distinction becomes clear, many plant behaviors that once seemed unpredictable begin to make sense.
This understanding is not advanced knowledge.
It is essential knowledge.
Clarifying the difference between heat and temperature removes one of the most common sources of confusion in thermal reasoning.
The next step is to recognize how deeply thermal effects shape everyday plant performance, even when the problem does not appear “heat-related” at first.
Why Most Plant Performance Issues Are Thermal
This article explains why many operational problems — fouling, instability, slow response, corrosion, and energy loss — are ultimately driven by heat transfer behavior rather than equipment defects or control settings.
